Which of the Following Has the Highest First Ionization Energy
Carbon has the highest first ionization energy. Between Rn and Pb the element with the higher first ionization energy is - 4.
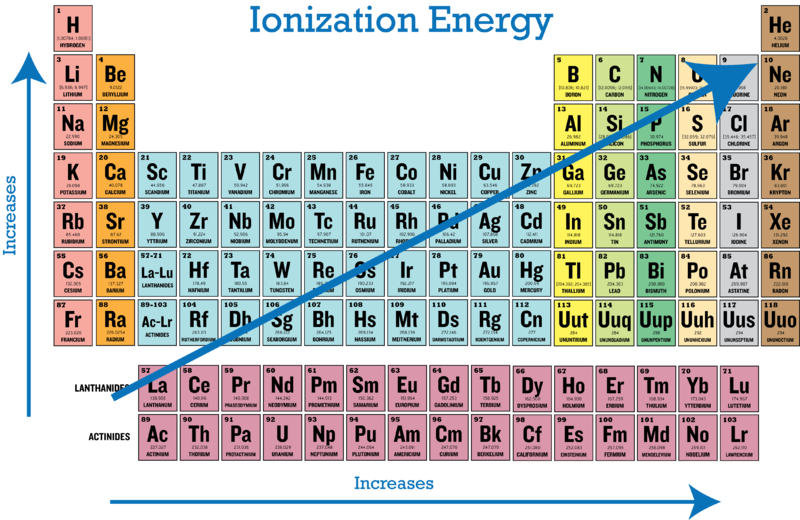
Periodic Trends In Ionization Energy Ck 12 Foundation
5441 eV 3.

. Hence less energy is needed to remove an electron. Cs At Ra Po. 120 rows First Second and Third Ionization Energy eV First Ionization Energy.
Asked 1 day ago in Chemistry by Sowaiba 712k points class-11. Of the following which element has the highest first ionization energy. Which of the following atoms has the highest first ionization energy.
The unity for ionization energy is eV. So energy required to remove the electron will be highest due to smallest distance between nucleus and valence shell. The order of the ionization energies is L i B e B C On moving from left t right in a period the ionization energy increases as the effective nuclear charge increases and the size decreases.
Core electrons efficiently shield outer electrons from nuclear charge b. Therefore we can conclude that Na atom has the highest first ionization. The elements that belong to the noble gases or inert gases or Group VIII-A have the highest ionisation energy.
Of the following which element has the highest first ionization energy. The first chemical element is Cesium and the last one is Helium. The elements that belong to the noble gases or.
Outer electrons efficiently shield one another from nuclear charge C. It mainly depends on the electrostatic attraction between the positive protons in the nucleus and negative electronsif protons and electrons are more attracted to each other then the first ionization energy would be higher and vice versa. Therefore sodium Na will have the highest ionization energy because it has the smallest size out of the given options.
The tabular chart on the right is arranged by Ionization energy. Ionization energy of Beryllium Be 932 eV. Between Kr and Br the element with the higher first ionization energy is - 2.
Hence option D is correct. Group of answer choices. Choose the statement that is true a.
Of the following which element has the highest first ionization energy. Since the nuclear charge is necessarily diminished with respect to the valence shell the alkali metals display the lowest ionization energies. Which has lowest first ionization energy.
Thus helium has the largest first ionization energy. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Further the ionization energy of carbon is 108645 Kj m o l 1.
Valence electrons are most difficult of all electrons to remove d. Ionization energy of Hydrogen H 1359 eV 2. The first ionization energy refers to the energy it takes to remove one electron from an atom.
Choose the element with the highest first ionization energy from each of the following pairs. So carbon has the highest first ionization energy. For chemistry students and teachers.
Of the following which element has the highest first ionization energy. If we were to take a single element then Helium is said to have the highest first ionization energy among all the other neutral elements. Ionization energy of Lithium Li 539 eV.
The ionization energy decreases from top to bottom in groups and increases from left to right across a period. The increasing order of the first ionization enthalpies of the elements BPS and F lowest first is. Of the following which element has the highest first ionization energy.
The first ionization energy of elements is inversely proportional to the atomic radii of elements. The ionization energy of an element is the energy required to remove one of the electrons in its outermost shell. The element with the highest first ionization energy among the choices is.
11 rows What Elements Have The Highest Ionization Energy. Ionization energy of Helium He 2458 eV. Between Mg and Ca the element with the higher first ionization energy is - 3.

Ionization Energy Periodic Trends

No comments for "Which of the Following Has the Highest First Ionization Energy"
Post a Comment